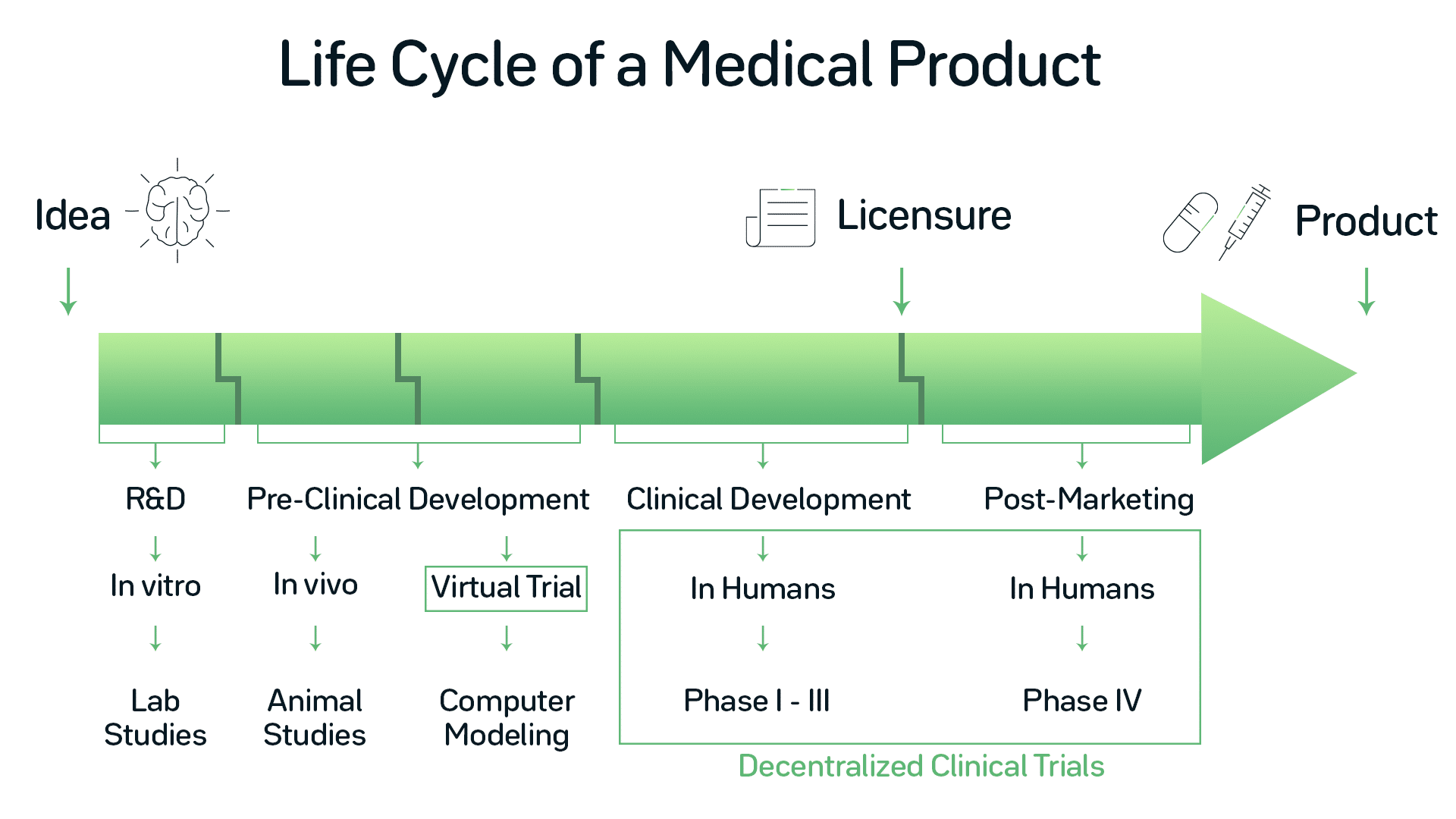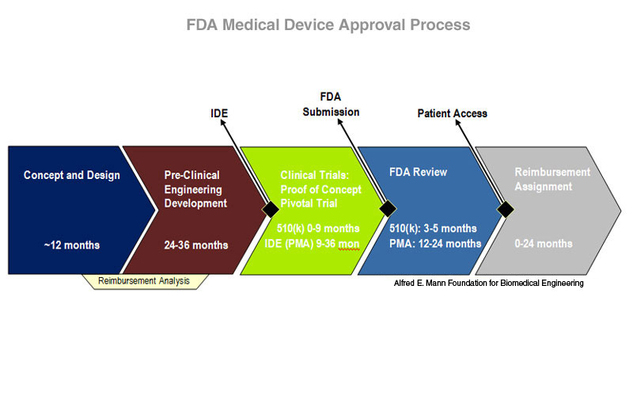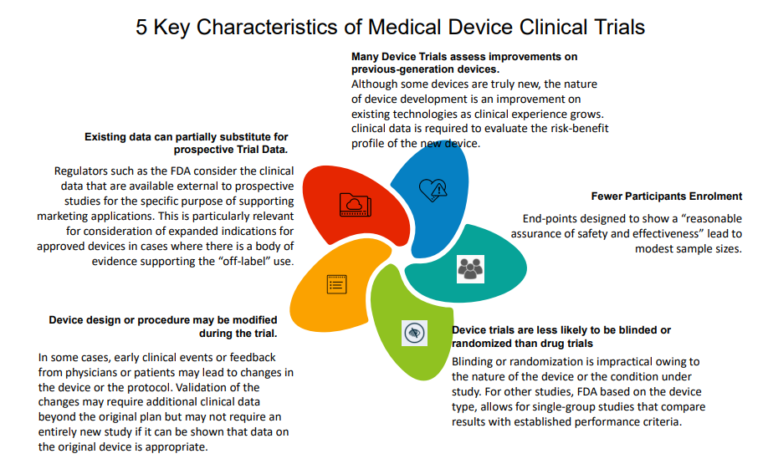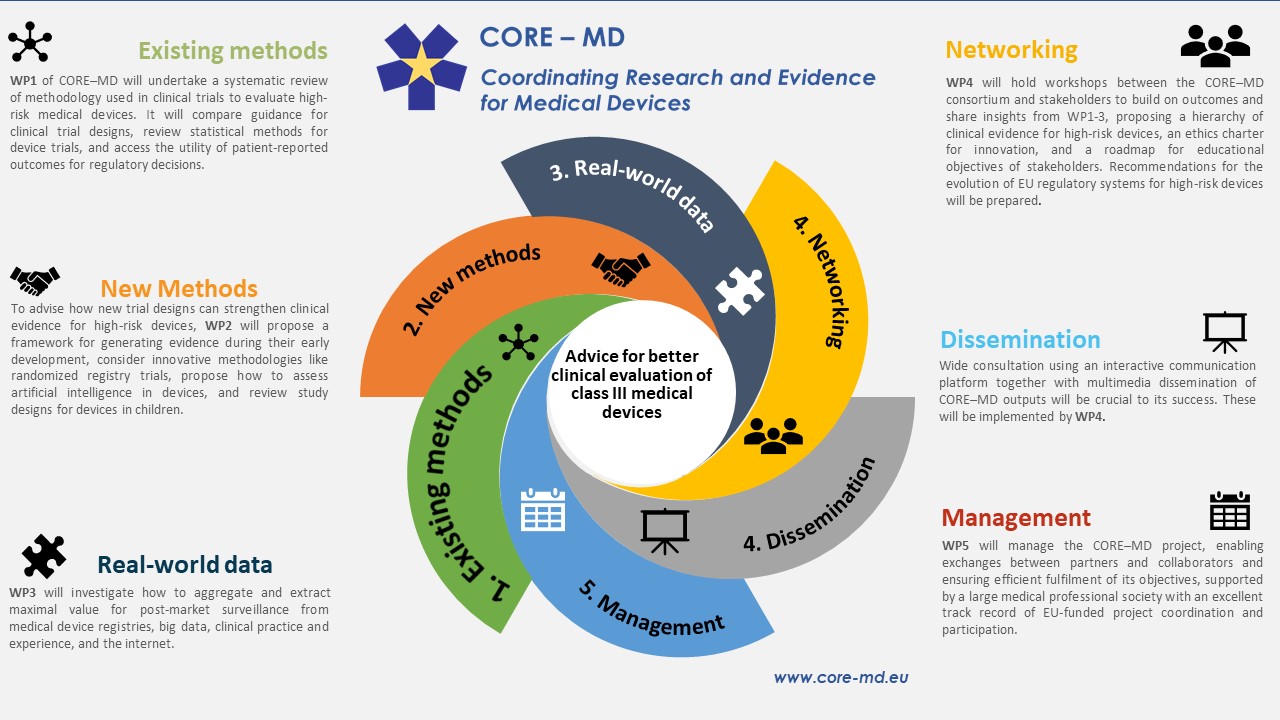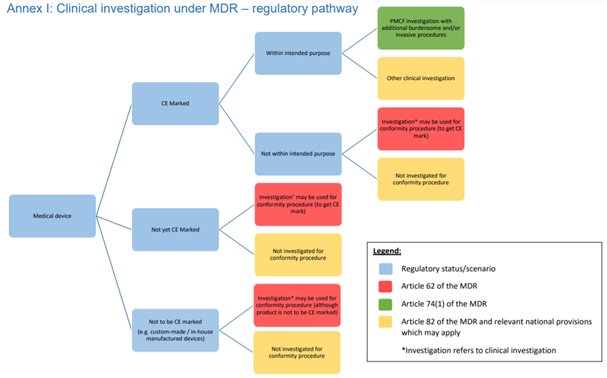
HOORAY: EU MEDICAL DEVICE CLINICAL TRIAL GUIDANCE ONE MINUTE TO MIDNIGHT! | Medical Devices Clinical

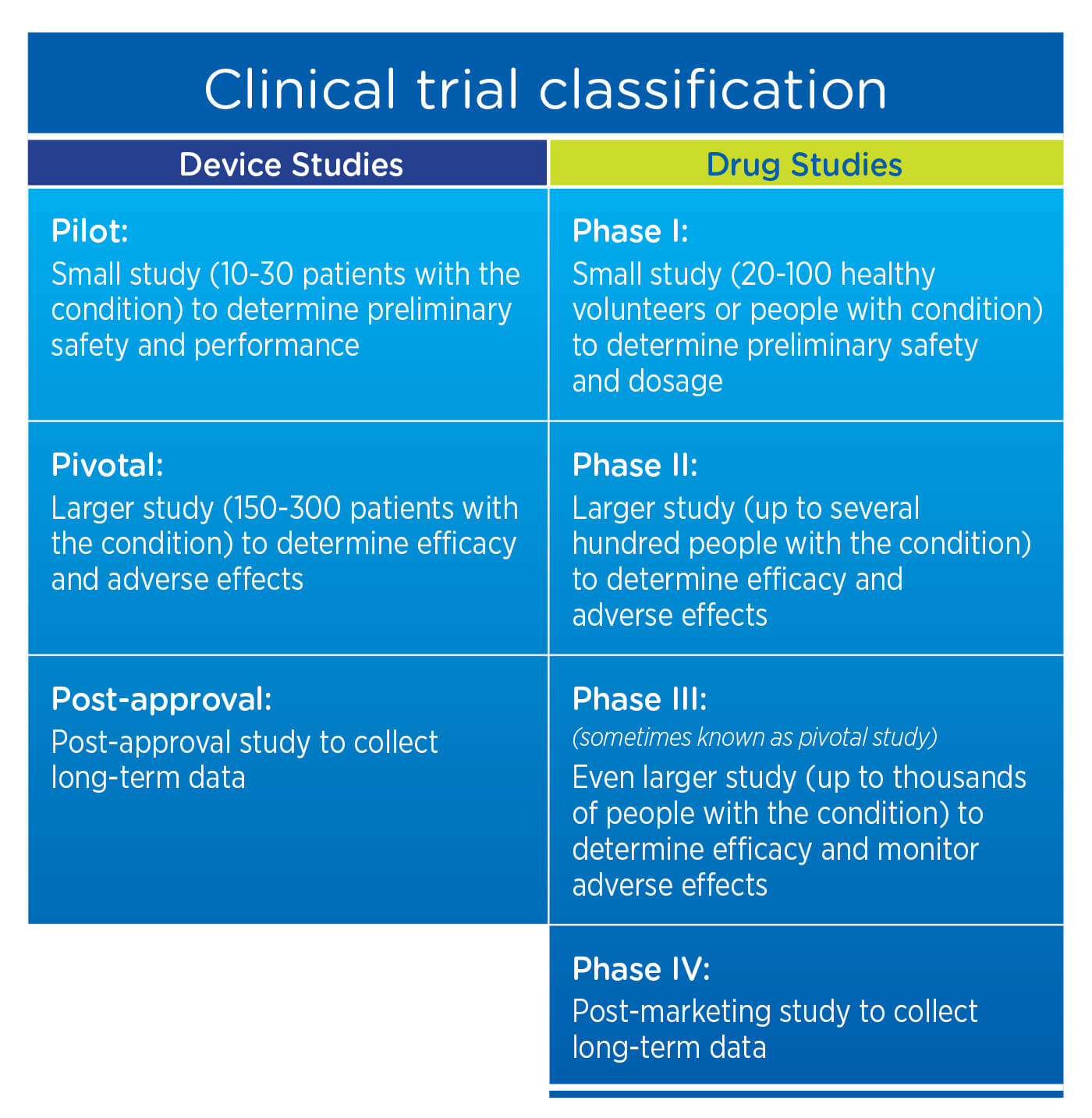
flowchart showing selection of clinical studies of new medical devices | Download Scientific Diagram
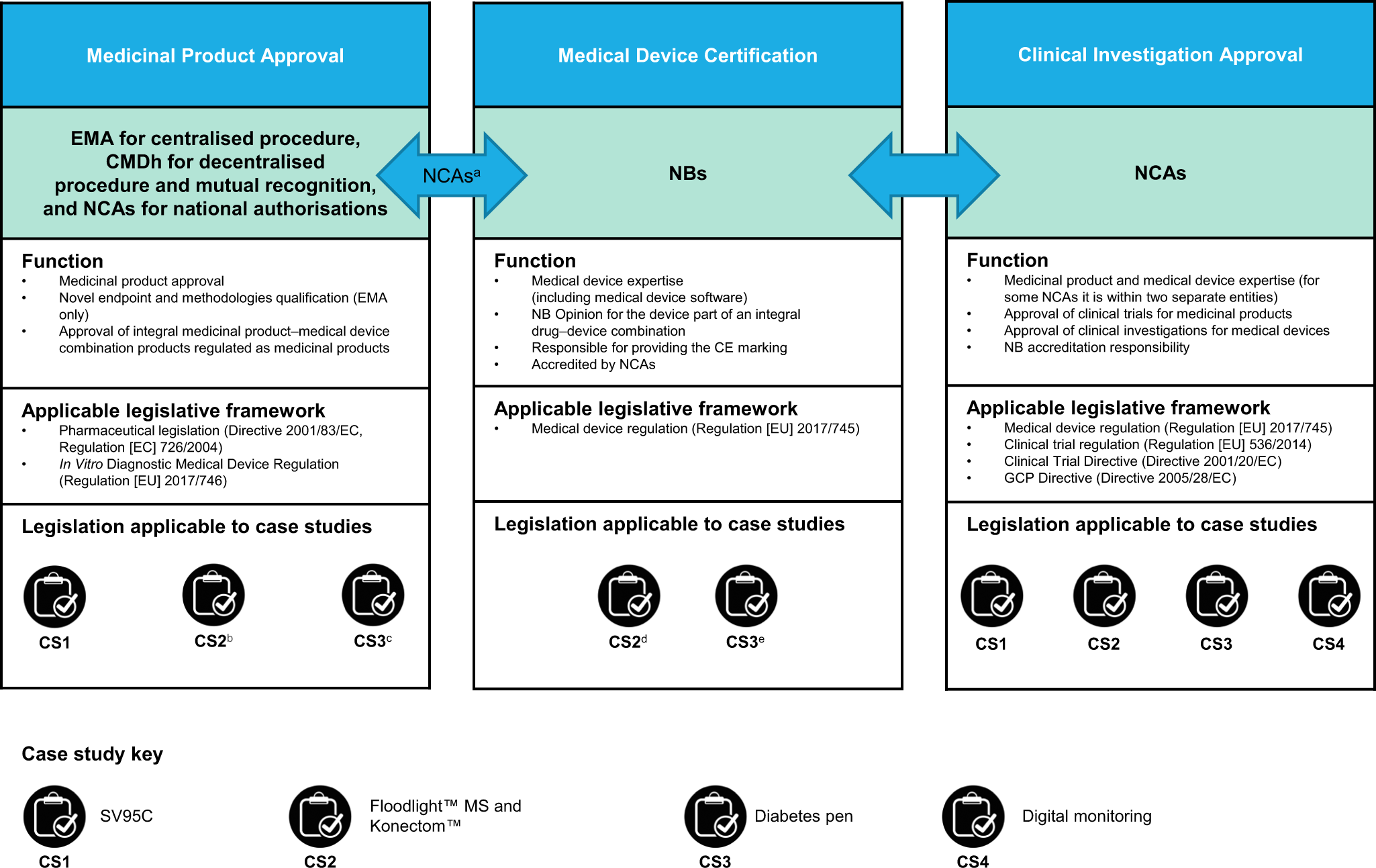
Evolving regulatory perspectives on digital health technologies for medicinal product development | npj Digital Medicine